A 6th revision post for the British Beekeeping Association’s Module 3 Honey bee Pests, Diseases and Poisoning exam, which I plan to take in March. I’m taking the BBKA’s correspondence course, so I have a tutor setting me papers which she then marks. I’m only on paper 2, the questions seem to go on forever…
3.a) describe the symptoms which might lead you to suspect the presence of the Acarine mite, Acarapis woodii, in a colony?
There are often no outward signs of the mite’s presence in a colony. Beekeepers should be suspicious if a colony does not build up properly in spring, as the mites shorten the lifespan of the bees (though nosema may be a more likely culprit). We should also be on their guard after poor summers, when bees are confined to the hive and mite transferal between bees is easy. Plenty of nectar means more flying bees and fewer opportunities to grab onto a new host. The result is that infested bees born in autumn will have their lives shortened whilst overwintering.
Sometimes acarine mites have been found on bees crawling or trembling by the hive entrance. This crawling may be caused by virus paralysis, but whether the mite acts as a vector for the virus or just happens to be present too is not known. Of course varroa is now present in virtually all UK hives and could also be acting as a vector, which complicates assigning blame. Crawling also happens for reasons unconnected with acarine or the paralysis virus.
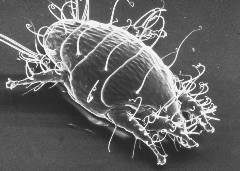
Acarine (tracheal mite)/small guinea pig. Courtesy The Food and Environment Research Agency (Fera), Crown Copyright.
b) how could you confirm the presence of this mite in the colony; include details of the size of the sample required, how the sample is obtained and how the bees are dissected as well as where the mite is normally found within the body of the bee?
Microscopic examination is required to confirm the presence of acarine.
The mite is usually found within the main thoracic trachea of the honey bee. Obtain a sample of around thirty bees. If crawling bees have been found unable to fly within about 3 metres of the front of the hive, collect these. Otherwise, try to collect foragers. The bees may be living, dying or dead. If alive, first kill them humanely with an ethyl alcohol solution or in a freezer at -20°C.
If attempting to analyse the sample yourself, pin the bee’s body on a wine cork through its thorax, between the second and third pair of legs. With a single edged razor blade or scapel, cut off the bee’s head and first pair of legs and remove them with tweezers. Next, carefully use fine tipped tweezers or a scalpel to remove its neck collar and fully reveal the main branches of the trachea. Pull the collar upwards in a circular motion; it should pull off easily, usually in one piece.
Image below from dave-cushman.net.

Inspect the bee’s trachea with a microscope at x40 magnification. You are looking for ‘staining’, blotchy dark patches caused as the mites damage the walls of the trachea when they pierce it with their mouth parts to feed on the bee’s haemolymph. Healthy tracheae are smooth and pearly creamy-white looking. Badly infested tracheae are discoloured from yellow to dark brown or black. If you are lucky the eggs, nymphs and adult stages of the mite may also be seen in the trachea.
Alternatively bee samples can be sent to the National Bee Unit in a sturdy card box (not plastic); for a charge of around £40 they will analyse the sample for you.

Courtesy The Food and Environment Research Agency (Fera), Crown Copyright. The healthy trachea is on the left side of the image, the acarine infested trachea on the right.
c) how does the mite spread from one colony to another?
Mites migrate to other bees as they touch. They do not seem to be able to transfer across comb or any other static object, so the most likely cause of transferal between colonies is worker bee drifting, drones paying visits to other hives or beekeepers moving combs containing adult bees between colonies.
d) write a short account about the life of the mite; include its lifecycle, what it feeds on, how and when the mite invades the body of the bee
The acarine mite (known as tracheal mite in the US) lives in the main thoracic trachea (respiratory tubes) of the honey bee. A fertilised female will find her way into a bee’s trachea and lay 5-7 eggs soon after the bee emerges from its cell – she prefers her host to be only one to two days old. There is some evidence that she prefers drones as hosts, due to their larger tracheas.
The eggs hatch about 3-4 days later, and after a period as immature nymphs the males larvae develop into adult mites within 11/12 days and the females within 14/15 days. The mite family feed on the haemolymph of the infested bee through the walls of the trachea, piercing it with their mouth parts. After becoming sexually mature adults, the brothers and sisters mate in the trachea and the fertilised females leave to find new hosts.
Impregnated adult female mites find a new host by leaving their original host’s trachea, clinging onto one of the bee’s body hairs with one or two hind legs, usually clustering under the wings first and then waving their remaining legs around until a suitable young bee (typically under three days old) comes along. They will be able to tell by the smell of the bee and also because the trachea is protected by hairs which are softer on young bees – the hairs across the spiracle quickly harden and prevent entry after the bee is a few days old.
The mite grabs the hair of her new host with her front legs (where she is probably attracted to the wing roots by their vibrations or by puffs of air (Pettis) from the first spiracles) and makes her way down the first thoracic spiracle to enter the trachea.
e) what is the effect of the mite on the individual bee.
- The breeding mites can eventually block the first pair of the bee’s tracheal tubes, shortening its foraging lifespan.
- Severely infested bees cannot fly and eventually die.
- Crawling and ‘K-wings’ may indicate infection with Chronic Bee Paralysis Virus (CBPV), which is often also present.
B4.a) in the debate over the cause of the so called Isle of Wight Disease, it has been suggested by Dr Bailey and others that many of the signs of this disease were not the direct result of Acarine but due to the presence of a secondary virus infection; discuss the reasons behind this assertion and name the virus infection implicated
In the early twentieth century, a mystery disease ravaged British bees. It was first observed in 1906 upon the Isle of Wight (for my American readers, this is a beautiful island about 5 miles off the south coast of England). Beekeepers there noticed that their bees were crawling on the ground around their hives, dying so fast that whole colonies were wiped out at the height of summer, when they should have been most strong.
The devastating affliction reoccurred at least three times from 1906 to 1919. By 1907 the disease had wiped out most of the bees on the island – it then spread to mainland England and wreaked havoc there. Huge numbers of bees had to be imported from Europe, so much so that some beekeepers claimed our darker British subspecies of Apis mellifera had effectively become extinct.
Looking back at 1906, when the disease first emerged, there was a gorgeously sunny April, drawing crowds to the Isle of Wight beaches. This was followed by an absurdly cold May – frosts and temperatures as low as -5°C (23°F), even in London. It was too cold for honey bees to venture out, at a time when colonies were full of young, spring bees. This created ideal conditions for a number of problems and parasites to take hold – such as dysentery (diarrhoea) due to the bees being unable to take cleansing flights. Of course if bees begin defecating on the combs this can spread Nosema, if it happens to be present. Acarine mites can also spread easily from bee to bee due to the number of bees squashed in together tightly.
Beekeeping in 1906 was very different to today. Beekeepers just did not have the same level of understanding about how diseases spread, with the result that all sorts of inadvisable practices were carried out, from keeping hives close together in apiaries to frequently moving combs and bees between different hives. These practices were recommended at the time, but must have helped spread the Isle of Wight disease. As travel and trade increased, it may be that the acarine mite first arrived in Britain through the Isle of Wight and Southern England at the start of the twentieth century. Meeting this parasite for the first time would have stressed colonies out more than we would now expect from acarine mites.
Although investigations in 1919 revealed the presence of acarine mites in all afflicted hives on the Isle of Wight, leading to the mites being identified as the likely culprit, it’s now thought that the crawling behaviour observed was probably due to Chronic Bee Paralysis Virus (CBPV). It was in the 1950’s that Dr Leslie Bailey (who worked in the Bee Disease Section of the Rothamsted Research Station) first suggested that CBPV was spread by the mite, with many of the colony losses in the 1910s ultimately being due to attack by this virus.
Edit – comment by Pam Hunter, 23/01/13: The combination of virus and acarine has a more profound effect than either alone. Viruses were not known at the time of the Isle of Wight disease but they can synergise with other infections. Now that we have varroa, this also synergises with acarine to have a far more profound effect than either alone.
Diseases and parasites such as nosema, acarine and varroa may not always kill colonies outright, but can weaken the immune systems of the bees, allowing viral infections to take hold. The descriptions given by beekeepers at the time of crawling bees with trembling wings we now identify with CBPV. It is only since the arrival of the electron microscope that scientists have been able to identify these viruses.
b) two main treatments for Acarine were advocated in the past. What were these treatments?
What a mean question! Now I have to memorise treatments that aren’t even used anymore?!
I think the answer is ‘Folbex‘ (Chlorobenzilate imregnated paper strips) and Folbex VA (Bromopropylate impregnated paper strips). The strips were set alight and allowed to smoulder in the hive, producing a smoke that penetrated through the hive. This treatment would be applied in the evening when all the foraging bees had returned; the hive would be closed up to hold in the smoke fumes and opened the following morning. In an out-apiary, the hive could be opened up after an hour so that the beekeeper did not have to return the next day.
Image below from dave-cushman.net.

There was also a treatment known as the ‘Frow‘ remedy, named after Richard Watson Frow MBE, a station master and amateur beekeeper. He published his treatment for others to read in the British Bee Journal’s 17 November 1927 issue. The remedy was based on a 1920s Daily News article on dealing with household pests.
It contained various dubious substances, Nitro benzene being involved in all formulations and generally the major constituent. Safrol, Ligrion, Petrol (gasoline) and Methyl Salicylate were all used as ingredients in different ratios and at different times. It was usually administered by giving small doses of about one millilitre on to a felt pad, daily or on alternate days, for a period six to ten days, then removing the pad after a further week to ten days.
To be fair, somehow the treatment worked, and Frow generously made the recipe freely available for beekeepers. For his contribution to beekeeping he was made a Member of the British Empire (MBE) in 1946. (This information on the Frow treatment comes from http://www.rogerparsons.info/frow.html and dave-cushman.net).
ii) why are they now not available?
Acarine is not considered a major disease in the UK, so there is no commercial incentive to produce a treatment.
The Frow treatment was highly inflammable and poisonous to bees and humans – two very good reasons why its use is not legal now! Its main component, Nitrobenzene, is highly toxic and possibly carcinogenic to humans. Inhalation of nitrobenzene vapors can cause headaches, nausea, fatigue, dizziness and in rare cases can even be fatal.
Edit – comment by Pam Hunter, 23/01/13: Both had a poor therapeutic ratio – i.e. the amount required to kill the mite was too close to the amount that would harm or even kill the bees.
c) if a hive of your bees has Acarine, what, if anything, could you do about it?
Currently there is no authorised treatment for acarine mites in the UK. The beekeeper may wish to consider requeening. Some honey bee sub-species are more susceptible than others – acarine is a bigger problem in the US, where many beekeepers use Italian/New Zealand crosses. Bees native to the UK appear to have evolved a tolerance to the mite.
Try to arrange hives in a way that minimises the risk of drifting. I have a blog post on doing this – honeybee drifting. Also try to discourage robbing – ideally feed bees inside their hives in the early morning or evening, when foragers are not out looking for food, and never feed bees communally in the middle of the apiary. Keep strong colonies with narrow entrances.
There is some evidence that thymol is effective against acarine, so using Apiguard or a similar thymol based authorised anti-varroa treatment might help. Edit – comment by Pam Hunter, 23/01/13: Some think that several years of using thymol has reduced acarine considerably.
In the US, some beekeepers have found feeding ‘grease patties’ to be effective. To make these, cooking oil is combined with white granulated sugar so that the sugar becomes oily and can be formed into a patty shape. A large spoonful is placed on greaseproof paper and placed on top of the brood combs, with an eke to give space. The idea is that young bees eating the patty will have their odour masked. This helps prevent the female acarine mites from recognising them as suitable young workers.
Photo below of a grease patty from tonitoni.org

Good husbandry techniques are also important. Inspect colonies regularly to make sure all is well and take samples for testing from any colonies that seem to be failing to thrive for no apparent reason.
d) if a colony died out from Acarine, is it safe to use the combs and other hive parts without the need for sterilization?
Yes, as acarine mites cannot survive on comb without the presence of bees. Eggs are only laid inside the bees’ trachea, not elsewhere in the hive. However, regular brood comb replacement and hive part sterilisation is good practice against several other pests and diseases, such as nosema, EFB and AFB.
References:
- Collins Beekeeper’s Bible, HarperCollins (2010)
- Dave Cushman’s website, ‘Treatment of Honey Bees for Acarine Mite‘
- Edinburgh Beekeepers, ‘Bee diseases and their treatment – Acarine‘
- Guide to Bees and Honey, Ted Hooper & Margaret Thomas, Northern Bee Books (2010)
- The Honey Bee Around & About, Celia F Davis, Bee Craft Ltd, (2009)
- Keeping Healthy Honey Bees, David Aston & Sally Bucknall, Northern Bee Books (2010)
- Mid Bucks Beekeepers Association, Module 3 exam notes
- National Bee Unit Beebase website
- Practical Beekeeping, Clive de Bruyn, Crowood Press (1997)
- Scientificbeekeeping.com, Randy Oliver


Poor things they seem to have to face a lot of challenges. I am really enjoying learning about the bees diseases.
LikeLike
Yes, there seems to be no end of things feeding on them. Plenty more disease posts to come!
LikeLike
another brilliant, useful post –
LikeLike
That is very kind of you to say, thanks! Does your blog have an RSS feed or email subscription btw?
LikeLike
I sent your posts on honeybee diseases and parasites to my son as he is a wildlife bio grad student and he finds the posts most interesting!!
How is the honeymoon planning going? My daugher is getting married on March 16!! Can’t believe it really. Seems like yesterday that she dressed a bride for her first grade Halloween party!! I will be a big puddle at the ceremony!!
Wondering what wonderful place you will settle on????
Cheers to you and thank your for your nice comments!!!
LikeLike
Thanks Cindy, glad your son enjoys them! Must be fascinating being a wildlife biology student.
How exciting that your daughter’s wedding is so soon! We are still looking, every weekend is taken up venue hunting! Btw I told one of my Twitter friends about your bird posts as he is a bird enthusiast. He said “Thank you, they’re great! The meadowlark is my favourite. The holler birds too — that funny woodpecker in the treetop!”
LikeLike
Good you can tell it is a woodpecker. I had no idea. A blogger identified it for me. The venue hunting sounds like lots of fun. Thank you for your wonderful comment and let me know where you end up going for your honeymoon.
LikeLike
Thanks Cindy, will do! 🙂
LikeLike