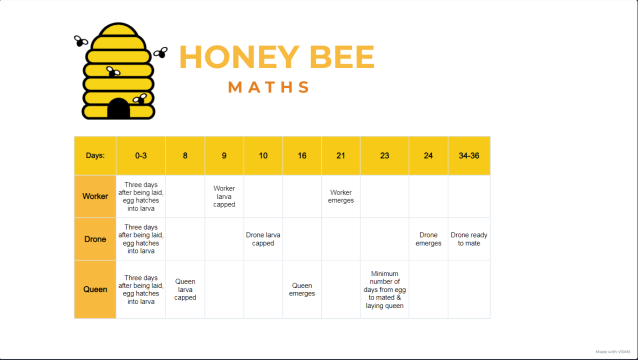
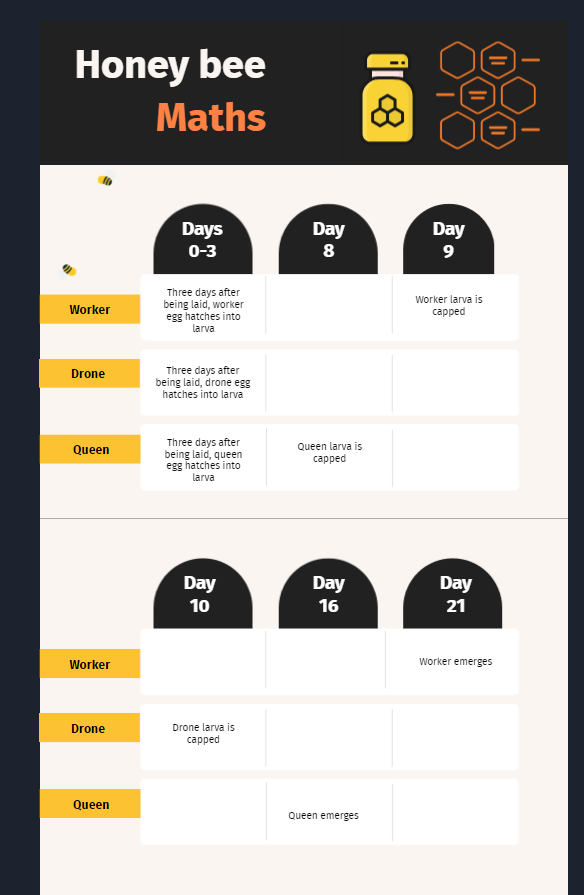
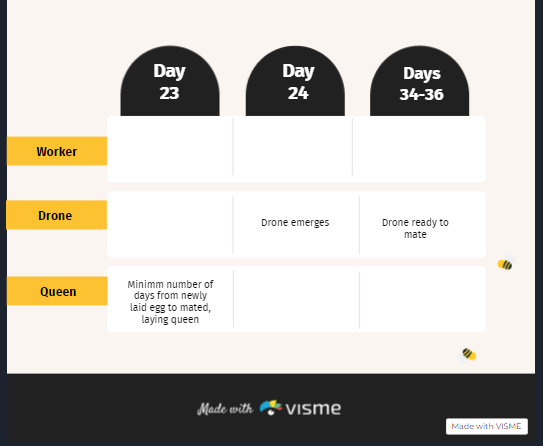
It’s been a long time since I last blogged. There’s been a lot going on.
But my house and garden is now pretty much how I want it, and I feel ready to write again now and then. Maybe even more than once a year!
One of my most successful plants this year has been this Erynigum planum, a close relative of sea holly. I bought from Rosy Bee plants, a specialist seller of plants suited to pollinators. Like foxgloves, it only flowers once every two years, so I am enjoying it while I can.
I am lucky as my front garden is south facing and gets sun nearly all day just outside my window. I’ve put out a bench and next to it this big pot with the Eryngium, where I can sit and watch the bees visiting it. Even from inside the house I can hear their buzz when the window is open! I was pleased to see this unusual looking bee come, a fast mover with a shiny black abdomen and fuzzy orangey-brown thorax. Helpful people on one of the UK bee facebook groups tell me it is Andrena thoracica, which mostly sticks to coastal areas around southern England and Wales.
Although this Erynigum planum plant is described as sea holly on the Rosy bee website, apparently it is actually a close relative of actual sea holly, which I have seen on the Cornish cliffs:
Another very popular plant with the pollinators in my garden just now is Verbena bonariensis (also sold at Rosybee, but they are not paying me commission!). I have lots of cabbage white butterflies visiting its tall open blooms and also bumble bees. A red tropical looking flower has self seeded next to it and I love the dramatic colour combo of red and purple.
Then a huge patch of geraniums has taken over one flower bed in the back garden now, but I don’t mind as they are pretty, flower for months and bees are over them all day.
Some of my garden visitors are easier to love than others, but I was impressed by this mother spider’s instincts. She stayed around her nursery web for a few days guarding it until the tiny baby spiders were big enough to disperse. Now the bees have the hebe plant all to themselves.
What flowers are the bees in your gardens enjoying right now? I’ve stopped keeping bees myself for the time being but have done a little beekeeping this year anyway, helping a friend who had this tiny swarm gathered around a mated queen – they would have about filled a coffee mug poor things.